Neuroinflammation in Glaucoma
Retinal neuroinflammation weakens mechanisms of ocular immune privilege
Glaucoma is characterized morphologically by retinal ganglion cell (RGC) death, degeneration of optic nerve axons, excavation of the optic nerve head, and degeneration of the lateral geniculate nucleus. While the changes in the optic nerve head are the clinical hallmark of glaucoma, it is the loss of RGC that is the ultimate cause of vision loss.
The degeneration of RGC in glaucoma is accompanied by a neuroinflammatory response, involving both retinal glia and RGC. Neuroinflammation is a necessary response of neuronal tissue to injury. Retinal neuroinflammation is one of the secondary events that result from RGC distress and degeneration in glaucoma.
While acute neuroinflammation generally does not result in adaptive immune responses, chronic neuroinflammation and activation of innate immune reactions cause the establishment of a pro-inflammatory milieu in the retina and link innate immunity to adaptive immune responses. For example, RGC damage is associated with activation of the complement cascade in the retina and release of the anaphylatoxins C3a and C5a. Both peptides are well-known to act as immune cell chemoattractants, modulate antigen presenting cell function, and increase blood-brain barrier (BBB) permeability. Furthermore, increased retinal levels of the cytokines IL-1 β and TNFα are well established events in the glaucomatous retina. TNFα, in addition to its apoptosis inducing effects, promotes lymphocyte diapedesis and is a potent stimulator of effector T-cells, while simultaneously inhibiting inflammation reducing T regulatory (Tregs) cells. Together, these pro-inflammatory signaling cascades degrade the immune privilege of the retina and fosters the activities of effector T-cells.
Interaction between neuroinflammation and systemic immunity in glaucoma
More recently we have made the observation that transfer of T-cells isolated from mice with glaucoma can induce retinal ganglion cell loss when transferred to normal, non-glaucomatous, mice. The recipient animals do not experience an elevation of intraocular pressure (IOP) and the loss of ganglion cells is progressive, slow, and specific i.e. no other neurologic or ocular effects are apparent. In a second studies have we demonstrated that lymphocytes significantly contribute to RGC loss in animal models of glaucoma. Induction of elevated IOP in RAG1-/- mice lacking mature T- and B-cells causes only approximately 50% of the RGC loss observed in immune competent mice. Moreover, unilateral induction of elevated IOP causes mild RGC loss in the contralateral eye in normal mice, but none in RAG1-/-, indicating that RGC damage in contralateral eyes is immune mediated.
These findings suggest that glaucoma induces a T-cell response that is sufficiently strong to cause damage to ganglion cells. If similar events also occur in glaucoma patients, it could explain why reduction of IOP does not always stop the progression of the disease since an autoimmune response, once initiated, could propagate ganglion cells loss in a completely IOP independent fashion. This would also indicate that immunomodulatory therapies may be able to prevent progressive vision loss in some glaucoma patients.
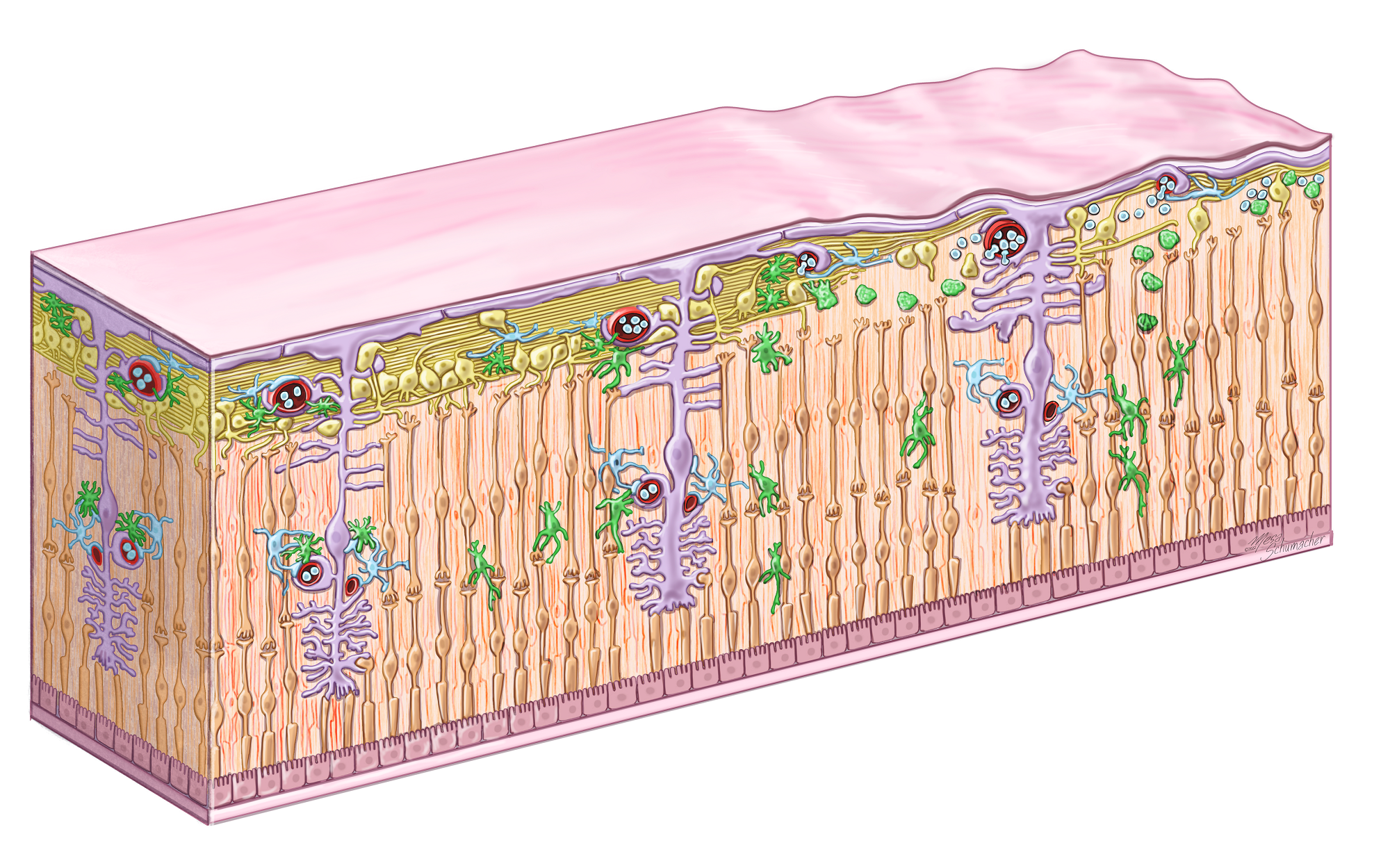
Cartoon illustrating the sequence of inflammatory events in the glaucoma retina. In the healthy retina (left) microglia (green) are ramified, astrocytes (purple) are not activated, and lymphocytes are contained within the vasculature. In early glaucoma (center) RGC stress causes glial activation and the secretion of pro-inflammatory cytokines. This both attracts lymphocytes and weakens the retina-blood barrier. In more advanced glaucoma (right) RGC loss is readily apparent and T-cells begin to extravasate. Continued interaction between T-cells and retinal glia then prevents a resolution of neuroinflammation in the tissue leading to continuous degeneration of RGC.
Last updated: 11/10/23
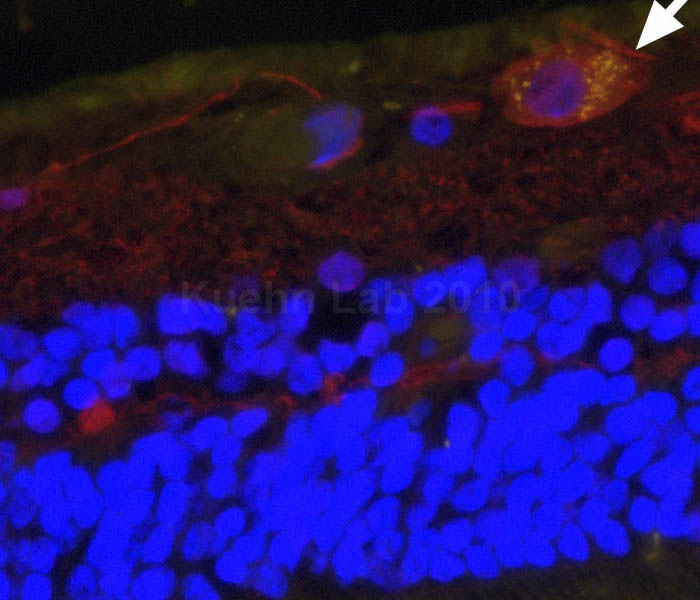
Retinal section showing deposition of the C5b-9 complement complex (yellow) on a retinal ganglion cell in a glaucoma eye.
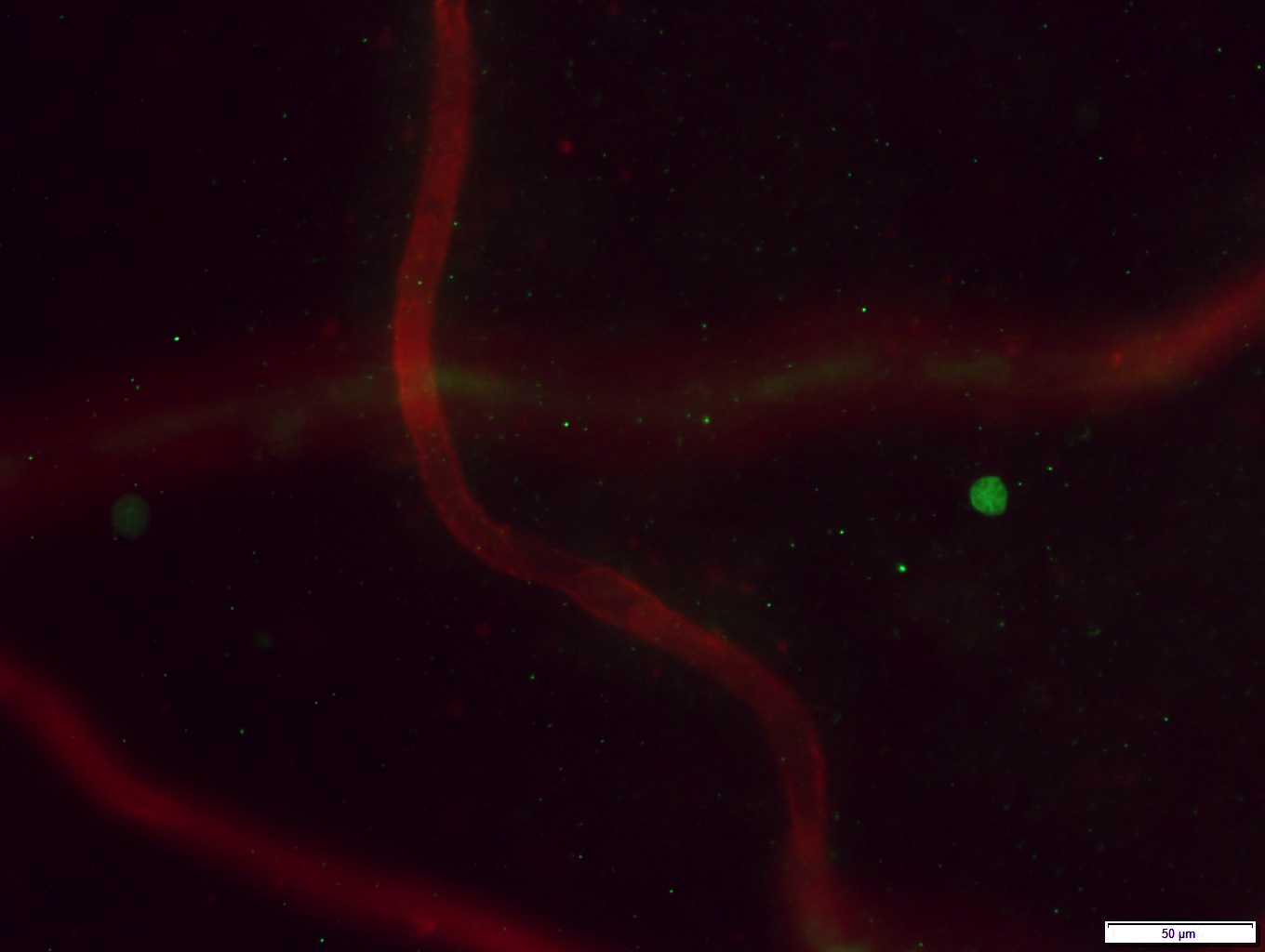
Microscopy image of a T-cell (green) that has left the blood vessels of the retina (red) in a human eye.